Introduction
FLT3 internal tandem duplication (ITD) is commonly detected in patients with acute myeloid leukemia (AML) and is known to confer poor prognosis. It has different insertion numbers, mutation burden, insertion sizes, insertion sites and insertion nucleotide sequences, showing complex diversity. However, the guideline of AML did not sufficiently consider the impact of the diversity of FLT3-ITD on treatment responses, and previous studies have not been consistent with the impact of FLT3-ITD diversity on clinical prognosis. In this study we examined the diversity of FLT3-ITD and correlated these parameters with clinical outcome in adult AML. In addition, we revealed the correlation between FLT3-ITD and drug resistance through evolutionary trajectories in primary resistant patients.
Methods
We included adult patients from 2016-2021 at Henan Cancer Hospital with newly diagnosed adult AML. next generation sequencing (NGS) was performed for all patients at diagnosis. The FLT3-ITD was also detected using conventional methods based on PCR, with a cut-off of 3% for positivity. The comparative analysis of NGS and PCR was done to validate the diversity of FLT3-ITD. Whole genome sequencing (WGS) data for multiple time points were used to analyze clonal evolution. Data were collected by chart review. The chi-squared or Fisher's exact test, Mann-Whitney U test, log-rank and Cox regression analyses were performed.
Results
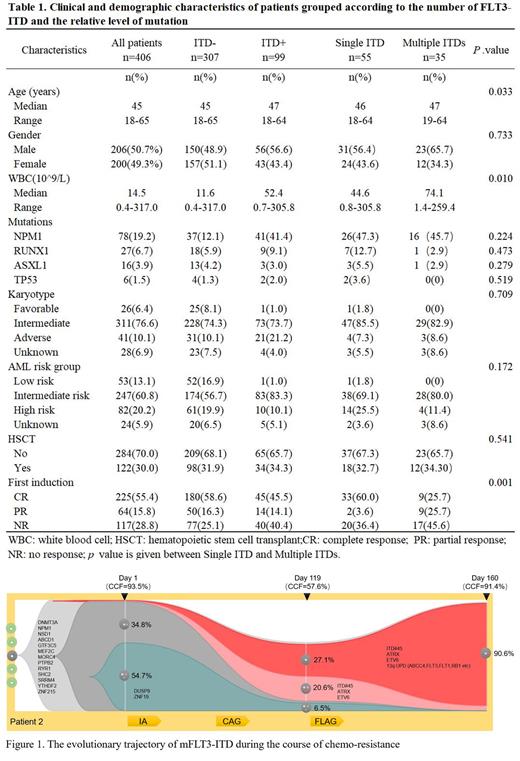
Among 406 newly diagnosed adult AML patients, 24.4% (99/406) patients were detected with FLT3-ITD. The FLT3-ITD diversity was validated by NGS and PCR data from 90 FLT3-ITD positive patients. 61.1% (55/90) patients had single FLT3-ITD (sFLT3-ITD) (31M/24F, median age 46 at diagnosis, range 18-64), and 38.9% (35/90) patients carried multiple FLT3-ITDs (mFLT3-ITD) at the same time (23M/12F, median age 47 at diagnosis, range 19-64). A total of 161 FLT3-ITDs were detected, with a median mutation burden of 21.51% (0.71-93.21%) and a median insertion length of 36bp (9-189 bp). 80.20% of FLT3-ITD insertions were in the near-membrane region, and 45.54% of FLT3-ITD insertions contained exogenous nucleic acid sequences.
Patients with mFLT3-ITD had a significantly lower CR rate than those with sFLT3-ITD (25.7% vs 60.0%, p=0.001), while the CR rate of patients with sFLT3-ITD was similar to patients without FLT3-ITD (60.0% vs 58.6%, p=0.521). In the setting of NPM1, the CR rate of patients with sFLT3-ITD+NPM1 mut improved significantly when compared to patients with sFLT3-ITD+NPM1 wild (76.9% vs 44.8%, p=0.015). Unfortunately, the state of NPM1 had no impact on the CR rate of patients with mFLT3-ITD. After a median follow-up of 38.9 months, the median overall survival (OS) has not been reached for patients with a single FLT3-ITD, and the median OS for patients with multiple FLT3-ITDs was only 12.4 months (95 CI, 3.8 to 20.9) ( p=0.028). Multivariate analysis confirmed its prognostic significance on CR rate (HR 2.13, 95% CI, 1.38-4.20, p=0.020) but not on OS. The mutation burden, insertion sizes, insertion sites and insertion nucleotide sequences of FLT3-ITD had no significant impact on the CR rate or OS.
To explore the clonal architecture of mFLT3-ITD, single cell DNA sequencing on one patient showed that respective FLT3-ITD variants were detected in completely separated cell populations. This suggested that mFLT3-ITD independently occurred in different subclone. WGS data of persistent primary resistant patients at multiple time points were used to analyze the evolutionary trajectory under chemotherapy. We observed complete separation of evolving trajectory of different FLT3-ITD variants over the course of chemo-treatment. Importantly, FLT3-ITD subclone of diagnosis evolved into the dominant clone at resistance.
Conclusion
In summary, by exploring the impact of the FLT3-ITD diversity on clinical prognosis and the evolutionary trajectory of mFLT3-ITD, the FLT3-ITD number may be a biomarker to refine the risk stratification and further identify adverse populations among FLT3-ITD positive AML patients.
Disclosures
Liu:Beigene: Speakers Bureau; Rigel: Speakers Bureau.